COVID-19 Vaccine Eligibility and Booking Guidelines

The North Durham Family Health Team is pleased to be included in the list of providers to offer COVID-19 vaccine to eligible patients. Appointments can be booked online by clicking the link below or by calling 905-985-2895 ext. 6070. We will not be accepting walk-ins at this time.
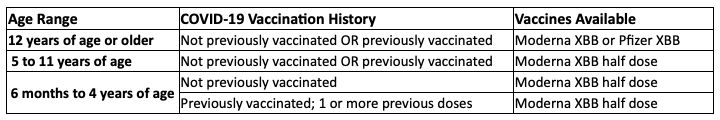
Please review eligibility criteria prior to booking your COVID-19 Vaccine appointment.
Clinics that we are currently running:
-
Moderna XBB Vaccines, for those 6 months and up, according to guidelines below
-
Pfizer XBB Vaccines for all booster doses for those 12+ years of age, according to guidelines below
Eligibility Criteria for COVID-19 Vaccines
All individuals who are 5 years of age and older and who have received at least one dose of any COVID-19 vaccine are eligible to receive an XBB dose this fall, if it has been 6 months (minimum 3 months) since their last COVID-19 vaccine dose or confirmed SARS-CoV-2 infection.
The XBB vaccine can also be given as a first dose vaccination with a shorter interval between doses.
Children can be given a half dose of the Moderna XBB as their first, second or subsequent doses.
By Clicking the booking link, you
COVID-19 Vaccine
Ready to book your COVID-19 vaccine? Click through below to start to process to booking an appointment with our medical staff online!